News & Insights
Sign Up for SoCalBio Newsletters
Stay informed about the events, news, and programs that are most important to your team.

Newsletter Archives
-
April 23rd, 2025
SoCalBio Weekly
-
April 16th, 2025
SoCalBio Weekly
-
April 9th, 2025
SoCalBio Weekly
-
April 2nd, 2025
SoCalBio Weekly
-
March 26th, 2025
SoCalBio Weekly
-
March 19th, 2025
SoCalBio Weekly
-
March 12th, 2025
SoCalBio Weekly
-
March 5th, 2025
SoCalBio Weekly
Special Reports
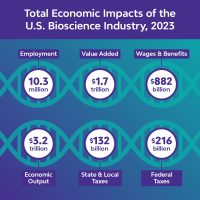
As part of the Council of State Bioscience Associations (CSBA), We are excited to announce the release of the 11th biennial Biotechnology Innovation Organization and CSBA report: “The U.S. Bioscience Economy: Driving Economic Growth and Opportunity in States and Regions”.